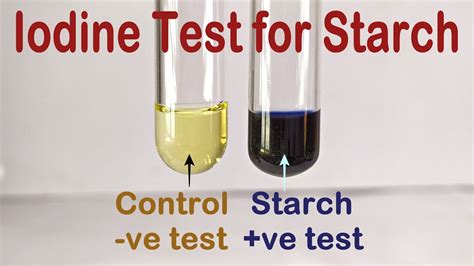
water iodine glucose starch permeability test|difference between iodine and glucose : white label To test whether iodine or starch have crossed the synthetic membrane, you will look for a change in color. A solution of iodine is tan and a solution of starch is clear or milky . WEBManhunt (с англ. — «Охота на человека») — компьютерная игра в жанрах стелс-экшен [4] от третьего лица и survival horror , разработанная британской студией .
{plog:ftitle_list}
Resultado da Definition of Alaguich in the Definitions.net dictionary. Meaning of Alaguich. What does Alaguich mean? Information and translations of Alaguich in the .
To test whether iodine or starch have crossed the synthetic membrane, you will look for a change in color. A solution of iodine is tan and a solution of starch is clear or milky .
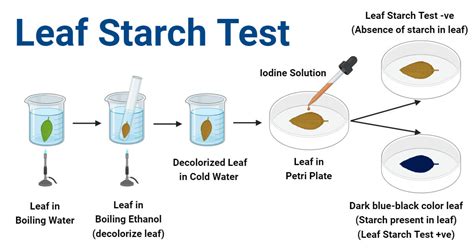
What can you conclude about the diffusion of iodine, glucose, and starch across the selectively permeable dialysis tubing membrane? Diffusion occurred based on molecular size. Iodine and .My Prediction: Iodine and glucose will be able to diffuse across the dialysis tubing membrane, but not starch due to its molecular size. My Strategy: I will place iodine on one side of the dialysis .3. Incubation: Place the dialysis bags in separate beakers containing distilled water and a few drops of iodine solution (iodine stains starch a dark blue/black). 4. Observation and Data . What can you conclude about the movement of Iodine, glucose, and starch across the dialysis membrane based on your results in Table 3? Support your answers for each with the observation from bag #5 and beaker #5.
Measure the diluted Starch/Glucose by placing a Glucose-testing strip in the solution, immediately removing it and waiting 60 seconds to observe any colour change. Using the colour guide on the testing strip container, determine the . This experiment investigates the permeability of cell membranes to various types of sugars: polysaccharides, disaccharides, and polysaccharides. Dialysis tubing is used to .
iodine vs starch testing
Pour approximately 50mL of water into a plastic sandwich bag and add 10mL of starch. Secure bag with the twist tie and shake gently to mix the starch. Put on gloves. Pour 250mL of water .Setup A: The starch will remain in the dialysis bag, meanwhile there will be movement of water molecules and iodine indicator into the bag. Setup B: The glucose will move out of the .Remove 1 ml of the water from the beaker and place in a test tube. Add 1 ml Benedict’s reagent to the test tube and place in the water bath for 5 minutes. Test the water in the beaker for starch by removing 1 ml of water from the beaker and placing it into a second test tube. Add 1 ml of iodine as an indicator.
The Iodine-Potassium Iodide Solution is used as an indicator for the presence of starch. Iodine (I 2) is relatively insoluble in water so potassium iodide (KI) is added to the solution; this results in the formation of iodide ions (I .the biological membrane (iodine), but not others (starch). QSA3. Iodine is an indicator solution that turns blue-black in the presence of starch. What process do you think occurred that caused the results you observed? Explain. Iodine is an indicator that turns blue-black in the presence of starch. If the two ingredientsExercise 1: Permeability of Dialysis Tubing Dialysis tubing is a semi-permeable membrane that excludes or permits molecules to cross based solely on their size. In this exercise, we will test the permeability of dialysis tubing to water, Lugol's iodine (IKI), glucose and starch. You will set up a model cell that contains 30% glucose and 1% starch.Starch plus iodine produces a blue-black color. So, if we add iodine to a solution . starch look like, so you are ready to proceed with an experiment to test the permeability of a membrane. . Beaker water - silver nitrate test iodine test (time 0 min) Experiment Now, place the ‘cell’ in the beaker, and record the time _____:_____ .
Benedict’s Test is used to test for simple carbohydrates. The Benedict’s test identifies reducing sugars (monosaccharide’s and some disaccharides), which have free ketone or aldehyde functional groups. Benedict’s solution can be used to test for the presence of glucose in urine. Some sugars such as glucose are called reducing sugars because they are capable of .
Suspend the tubing in a beaker of water for a set period of time; Take samples from the liquid outside of the visking tubing at regular intervals and test for the presence of starch and glucose. Starch is tested for using iodine. A blue-black colour is produced in the presence of starch; Glucose is tested for using Benedict's reagent. Iodine Test . Test glucose, fructose, lactose, sucrose, starch, water, and compare with a sample of a solution with an unknown component. Add 1 mL of each solution to be tested to each of 7 labeled test tubes. Add 3 drops of iodine solution to each of the 7 test tubes, and mix each tube. Compare the colors and record your observations.iodine, starch, and a dropper Procedure v 1.) Fill the plastic bag with 40 mL of starch solution. Twist the top of the bag and tie it. 2.) Observe and record the color of the starch in the before section of the data table. 3.) Fill a beaker with 80 mL of water, and add iodine to the water until the water is a golden yellow color. 4.)Measure the diluted Starch/Glucose by placing a Glucose-testing strip in the solution, immediately removing it and waiting 60 seconds to observe any colour change. . Fill a large beaker with 100mL water, and add 1mL of Iodine/KI solution. The solution should appear a yellowish colour. Measure the Glucose levels of the Iodine solution with .
Starch consists of long chains of glucose (atomic mass of each glucose = 180). Iodine turns a deep blue in the presence of starch. Formulate a hypothesis for each of the following. Remember to provide a reasonable explanation for your predictions. The movement of starch; The movement of iodine; The color of the solution in the bag after 30 minutes Other substances, like glucose or sodium ions, are unable to pass through the cell membrane unless they are specifically transported via proteins embedded in the membrane itself. . Fill beaker #2 with 300 ml of tap water, then add iodine drops drop by drop until the solution is bright yellow. Now prepare your 2 dialysis tubing “bags .
The discrepancy in permeability is due to the difference in the sizes of iodine and starch molecules. Permeability of cell model to lactose The Benedict’s test control on lactose yielded a solution that was a murky yellow-brown color; this indicated the presence of a mono- . Another telling observation was the finding that glucose permeability through erythrocyte membranes is a million times greater than that through an artificial lipid bilayer. The concentration of glucose in the blood is relatively high compared to that inside of most cells, so this is mediated transport, but passive transport since it is going .place glucose and starch inside dialysis tubing and then place the tubing in a beaker containing iodine and water-then observe and record observations after 10 minutes. . glucose test strips. media. substances through which diffusion occurs, such as water and air.Experiment 2: Concentration Gradients and Membrane Permeability The positive control for the IKI solution was [a] and the resulting color was [b]. The positive control for the Glucose Test Strip was [c] and the resulting color was [d]. The negative .
This video is about Diffusion of Water, Glucose, and Starch through a Dialysis Bag
The test tube with water will be the control or comparison sample. Test for Cl - by adding 1 drop of AgNO3 to each. Record your observations. Compare the results for the NaCl solution with the results of the water sample. Glucose test: Place 1.0 mL of 10% glucose solution in a test tube. Place 1.0 mL of DI water into another test tube. Add 1.0 .12. For test tubes 1,2 and 4, both A and B, add a benedict’s solution and mix. 13. Place the test tubes in boiling water and observe. 14. If the color changes to yellow, green, or brown then the solution is positive for sugar, record the results. 15. For test tubes 3A an d B, add silver nitrate solution dropwise. 16.
Other substances, like glucose or sodium ions, are unable to pass through the cell membrane unless they are specifically transported via proteins embedded in the membrane itself. . Fill beaker #2 with 300 ml of tap water, then add iodine drops drop by drop until the solution is bright yellow. Now prepare your 2 dialysis tubing “bags .The experiment investigated the permeability of dialysis tubing to glucose, Iodine potassium iodine, and starch. The color of bag (containing glucose and starch) before the benedict's test was blueish and after the benedict test the color was yellow. The color of becker (containing water and iodine) was clear before the Benedict's test and .
Using iodine to test for the presence of starch is a common experiment. A solution of iodine (I 2) and potassium iodide (KI) in water has a light orange-brown color. If it is added to a sample that contains starch, such as the bread pictured above, the color changes to a deep blue. But how does this color change work? Starch is a carbohydrate .
The iodine–starch test was first described in 1814 by Jean-Jacques Colin and Henri-François Gaultier de Claubry, [3] and independently by Friedrich Stromeyer the same year. [4] [5]In 1937, Canadian-American biochemist Charles S. Hanes extensively investigated the action of amylases on starch and the changes in iodine coloration during starch degradation and proposed a .
Principle Of Iodine Test For Starch The starch-iodide complex as charge is transferred between the starch and iodide ions (tri-iodide or pentaiodide). The transfer of the charge between the starch and the iodide ion changes the spacing between energy levels/orbitals. This change results in the starch-iodide complex absorbing light at different .Chemical Test for Starch or Iodine. Amylose in starch is responsible for the formation of a deep blue color in the presence of iodine. The iodine molecule slips inside of the amylose coil. Iodine - KI Reagent: Iodine is not very soluble in water, therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide.The iodine and water solution in the beaker was a clear yellow color. A Benedict’s test on the beaker solution after the experiment yielded a dark brown liquid; a Barfoed’s test on the beaker solution after the experiment resulted in a clear blue liquid. Discussion Permeability of cell model to .
iodine vs starch protocol
web24 de out. de 2023 · Based on the fantasy bestseller: To save humanity from the Gryphon’s claws, outcast Mark must confront his family’s dark legacy and enter a deadly battle in the .
water iodine glucose starch permeability test|difference between iodine and glucose